I don't usually just post a link to an article, but the following is worth reading. The author is Tony Newman, of the Drug Policy Alliance.
Drug Users of the World Unite

Opiophilia is a love of opioids, This blog is devoted to everything in the world of opioids, and the people who love them. It is also my wish to portray an accurate picture of opioid users, the good, bad and ugly, without hysterics or demagoguery. Also included a is information on harm reduction, opinion pieces and the occasional rant.
Monday, December 31, 2012
Friday, December 21, 2012
How Heroin is Made
 This review of a heroin manufacturing process uses images from Afghanistan, though opium poppies grow all over the world and the process of making heroin destined for the black market is the same. It all starts here with the harvesting of opium from the poppy. The pods are lightly scored with a blade and the raw opium collected. The opium is collected into balls and transferred to a makeshift "laboratory" where the morphine will then be extracted and processed into heroin.
This review of a heroin manufacturing process uses images from Afghanistan, though opium poppies grow all over the world and the process of making heroin destined for the black market is the same. It all starts here with the harvesting of opium from the poppy. The pods are lightly scored with a blade and the raw opium collected. The opium is collected into balls and transferred to a makeshift "laboratory" where the morphine will then be extracted and processed into heroin. Upon arrival at the site the opium is unpacked and placed into tubs. It is then crushed and divided into portions for each batch. The crushed opium was then placed into barrels and hot (not boiling) water is added. At this point the pH is around 8. The barrel is then stirred and any solids that float to the surface, pieces of plastic or plant matter, are removed. Hot water with lime (Calcium Oxide, CaO) is added until all the opium has dissolved and the pH reaches 10-12. The barrels are then filled with water, covered and left to sit overnight. The following day the insoluble oils and resins will be floating on the surface, the opium solution containing the morphine is then siphoned out into separate containers.
Upon arrival at the site the opium is unpacked and placed into tubs. It is then crushed and divided into portions for each batch. The crushed opium was then placed into barrels and hot (not boiling) water is added. At this point the pH is around 8. The barrel is then stirred and any solids that float to the surface, pieces of plastic or plant matter, are removed. Hot water with lime (Calcium Oxide, CaO) is added until all the opium has dissolved and the pH reaches 10-12. The barrels are then filled with water, covered and left to sit overnight. The following day the insoluble oils and resins will be floating on the surface, the opium solution containing the morphine is then siphoned out into separate containers.  The dark brown opium solution which contains the morphine is siphoned off from the insoluble oils and residues in the opium. The solution may then be filtered again through cloth or sacks to further remove insoluble particles. The filtrate (liquid portion) is then poured back into a barrel.
The dark brown opium solution which contains the morphine is siphoned off from the insoluble oils and residues in the opium. The solution may then be filtered again through cloth or sacks to further remove insoluble particles. The filtrate (liquid portion) is then poured back into a barrel.  At this point the morphine is ready to be precipitated (solid morphine comes out of the liquid) by the addition of Ammonium Chloride (NH4Cl) while stirring continuously. After all the ammonium chloride is added, the barrel is covered and left to stand overnight.
At this point the morphine is ready to be precipitated (solid morphine comes out of the liquid) by the addition of Ammonium Chloride (NH4Cl) while stirring continuously. After all the ammonium chloride is added, the barrel is covered and left to stand overnight.
The following morning the contents of the barrel are poured into a bucket lined with cloth soaked in warm water. After being poured through the bucket the morphine base is wrapped in the filtering cloth and squeezed to remove remaining liquid. The brownish-grey morphine base is then spread out on a cloth to air-dry in the sun. The dried morphine base is then weighed in preparation for the acetylation reaction to convert the morphine to heroin. This crude morphine base is about 50% morphine, 20% narcotine and 30% other compounds.

The crude morphine base is placed in an aluminum pot and a slight excess of acetic anhydride is added to the pot. The pot is stirred until all the morphine has dissolved and then left for 45 minutes. A fire is built during this time and after the 45 minutes have passed the pot is heated for 30 minutes.
 The reaction mixture is then poured into a bowl with warm water and the solution is filtered again into another barrel. A solution of sodium bicarbonate (baking soda) is poured into the barrel, carbon dioxide gas is given off as the base reacts with the acid.[1] When the solution no longer gives off gas and the pH reaches 10 the crude heroin base will be precipitated out of the solution.
The reaction mixture is then poured into a bowl with warm water and the solution is filtered again into another barrel. A solution of sodium bicarbonate (baking soda) is poured into the barrel, carbon dioxide gas is given off as the base reacts with the acid.[1] When the solution no longer gives off gas and the pH reaches 10 the crude heroin base will be precipitated out of the solution. The solution is then filtered again through a cloth. The brownish colored crude heroin is then poured into a bowl to be prepared for the final purification step.
The solution is then filtered again through a cloth. The brownish colored crude heroin is then poured into a bowl to be prepared for the final purification step.  The brown heroin base is then dissolved in dilute hydrochloric acid until the pH reaches 7-8. Activated carbon is added to the solution and left to sit for 30 minutes. The solution is then filtered again through a cloth, and a second time through a filter paper. After the impurities are removed, the heroin base is precipitated out by addition of a dilute ammonia solution until the pH reaches 12. The solution is filtered again, leaving a relatively pure, white heroin base.
The brown heroin base is then dissolved in dilute hydrochloric acid until the pH reaches 7-8. Activated carbon is added to the solution and left to sit for 30 minutes. The solution is then filtered again through a cloth, and a second time through a filter paper. After the impurities are removed, the heroin base is precipitated out by addition of a dilute ammonia solution until the pH reaches 12. The solution is filtered again, leaving a relatively pure, white heroin base. The final step involves dissolving the heroin base in a hydrochloric acid and acetone solution. The solution is filtered through a filter paper into a metal bowl. The liquid is evaporated leaving off-white heroin hydrochloride. This final product is about 75% pure, see Table 4 below. The yield of heroin from raw opium, is about 6% by weight.
The final step involves dissolving the heroin base in a hydrochloric acid and acetone solution. The solution is filtered through a filter paper into a metal bowl. The liquid is evaporated leaving off-white heroin hydrochloride. This final product is about 75% pure, see Table 4 below. The yield of heroin from raw opium, is about 6% by weight.
The process of making heroin from opium is a simple one and can be done anywhere in the world. The following two tables list the needed equipment and chemicals needed for the process. Calling these laboratories is generous, and truly an insult to real chemical labs. For these reasons it should be obvious that there will never be a victory in the war against heroin by attempting to stop the supply at its source. Opium poppies grow all over the world and the chemical reaction requires only the most primitive equipment. Electricity and running water is not even needed. The only chemical that would be difficult in any way to procure is the acetic anhydride.
The intensified effort to block the Taliban's access to acetic anhydride comes as the Afghan war turns deadlier, with a record 236 U.S. and allied troops killed so far this year, according to icasualties.org, a Web site that tracks coalition fatalities. U.S. Army General John Craddock, supreme allied commander for Europe, is pushing to increase military involvement in countering the narcotics trade.
The chemical allows Afghanistan's drug lords to dramatically increase revenue by producing heroin in their own laboratories instead of shipping out raw opium to be processed elsewhere. According to Craddock, a kilo of opium fetches about $100, compared with $3,500 for heroin.
This article is from 2008, so apparently that "rare" victory was short-lived. Given the 6% yield of heroin from opium, it would take 16.7 kg of opium to produce 1 kg of heroin. Using the figures provided by Craddock, to produce a kilogram of heroin requires $1,670 worth of opium to produce a product worth $3,500, more than doubling the value. Additionally the final product is only 6% the weight, making it easier to smuggle to destination countries. Compared to the logistics of smuggling thousands of tons of opium out of the country to be processed elsewhere, it is far easier to smuggle the acetic anhydride into the country than opium out.

Many of the images and information in this post were taken from the following document:
Documentation of a heroin manufacturing process in Afghanistan. U. Zerell, B. Ahrens and P. Gerz. Federal Criminal Police Office, Wiesbaden, Germany [Link]
Wednesday, December 19, 2012
Conflating the Harms of Heroin with the Harms of Prohibition
I have a problem with this graph, from a 2007 Lancet article by David Nutt. I actually like David Nutt, he was fired from his position in the UK government for criticizing the decision to reclassify cannabis to Class B from C. That's right, a scientist who was working for the UK equivalent of the NIDA actually bucked the orthodoxy of "drugs are bad, mmkay," of course he was sacked for it. You will notice that heroin scores the highest on both dependence and physical harm. This the criteria used to evaluate physical harms in the lancet article:
Assessment of the propensity of a drug to cause physical harm—ie, damage to organs or systems—involves a systematic consideration of the safety margin of the drug in terms of its acute toxicity, as well as its likelihood to produce health problems in the long term. The effect of a drug on physiological functions—eg, respiratory and cardiac—is a major determinant of physical harm. The route of administration is also relevant to the assessment of harm. Drugs that can be taken intravenously—eg, heroin—carry a high risk of causing sudden death from respiratory depression, and therefore score highly on any metric of acute harm. Tobacco and alcohol have a high propensity to cause illness and death as a result of chronic use. Recently published evidence shows that long-term cigarette smoking reduces life expectancy, on average, by 10 years.9 Tobacco and alcohol together account for about 90% of all drug-related deaths in the UK.My main problem with the lancet article is they compare legal drugs, alcohol and tobacco, with illegal ones like heroin. Black market heroin is a dangerous drug because of its unknown purity and possibility of being adulterated with any number of substances, assuming you're even getting heroin and some some obscure fentanyl analog. Some basic information would go a long way to making heroin a less dangerous drug. For one combining opiates with CNS depressants dramatically increases the chance of overdose. Easy access to naloxone (narcan) nasal sprays could save countless lives, without having to call 911 and risk arrest in states without "good samaritan" laws. Disseminating information on safe injecting practices would go a long way to improving users health. Instead we get PSAs with a teenage girl destroying her kitchen in an apparently heroin-fueled rage. Furthermore due to the social stigma of using heroin, most users hide their drug use making it more likely no one will be around to save their life should they OD.
This is comparing apples to oranges. To compare apples to apples one would have to look at a quasi-legal system. One option would be to compare the physical harms of individuals on heroin prescriptions or physician opiate users. Both use pharmaceutically pure opiates and sterile injecting equipment. In the heroin assisted therapy trials in Vancouver there were ODs, but medical staff quickly revived them. Most importantly there were no opiate-related fatalities. Putting aside the potential for an opiate overdose, just how bad for the body is heroin? Dr. Arnold Trebach, writing in "The Heroin Solution," informs us that "putting aside the problem of addiction, the chemical heroin seems almost a neutral or benign substance. Taken in stable, moderate doses, it does not seem to cause organic injury, as does alcoholism over time." Interesting, so if heroin were administered in an environment where the chance of ODs were minimized, and with a readily available narcotic antagonist (antidote), heroin would score below alcohol and tobacco, probably just above cannabis. Heroin is the poster child for prohibition making drug use far more dangerous than it would be in a legal, regulated market.
Rob Arthur writing in his article, "Heroin Is Harmless?" breaks it down the harms further:
Three aspects of an ingestible substance that can be considered harmful are (1) its potential to debilitate, (2) its effects on one’s health, and (3) its potential to kill via an overdose.
(1) Like the stimulants, caffeine and cocaine, heroin is not a debilitating drug. That is, moderate usage does not interfere with one’s functioning, e.g. driving ability. This is in contrast to alcohol, in which one’s performance is directly hampered. Extreme usage can interfere just like with caffeine and cocaine, e.g. too much of a stimulant can make it difficult to focus and even cause hallucinations. However, even heroin addicts can moderate their usage so that they can work unimpaired and avoid withdrawal symptoms. For this reason, heroin addicts can and do have successful professional lives in such diverse fields as surgery and law enforcement.
(2) Long-term heroin addiction is relatively harmless to one’s health. Like caffeine addicts who “need” their coffee in the morning, the side-effects are minimal. Heroin’s long-term side-effects can include constipation and impotency. This is in contrast to alcohol and tobacco which destroy the liver and the lungs respectively.
(3) Like caffeine, it is difficult to fatally overdose on heroin by itself. (It is easy to overdose when using heroin and alcohol in combination.) The popular image of a dead heroin user with the needle still in his or her arm is misleading. A fatal heroin overdose is usually a long process that takes over an hour and it can be countered within minutes by an antidote.
Friday, December 14, 2012
HSBC Launders Drug Money, Two Articles
Outrageous HSBC Settlement Proves the Drug War is a Joke by Matt Taibbi
The banks' laundering transactions were so brazen that the NSA probably could have spotted them from space. Breuer admitted that drug dealers would sometimes come to HSBC's Mexican branches and "deposit hundreds of thousands of dollars in cash, in a single day, into a single account, using boxes designed to fit the precise dimensions of the teller windows."
This bears repeating: in order to more efficiently move as much illegal money as possible into the "legitimate" banking institution HSBC, drug dealers specifically designed boxes to fit through the bank's teller windows. Tony Montana's henchmen marching dufflebags of cash into the fictional "American City Bank" in Miami was actually more subtle than what the cartels were doing when they washed their cash through one of Britain's most storied financial institutions.
[...]
So you might ask, what's the appropriate financial penalty for a bank in HSBC's position? Exactly how much money should one extract from a firm that has been shamelessly profiting from business with criminals for years and years? Remember, we're talking about a company that has admitted to a smorgasbord of serious banking crimes. If you're the prosecutor, you've got this bank by the balls. So how much money should you take?
How about all of it? How about every last dollar the bank has made since it started its illegal activity? How about you dive into every bank account of every single executive involved in this mess and take every last bonus dollar they've ever earned? Then take their houses, their cars, the paintings they bought at Sotheby's auctions, the clothes in their closets, the loose change in the jars on their kitchen counters, every last freaking thing. Take it all and don't think twice. And then throw them in jail.
Sound harsh? It does, doesn't it? The only problem is, that's exactly what the government does just about every day to ordinary people involved in ordinary drug cases.
[...]
On the other hand, if you are an important person, and you work for a big international bank, you won't be prosecuted even if you launder nine billion dollars. Even if you actively collude with the people at the very top of the international narcotics trade, your punishment will be far smaller than that of the person at the very bottom of the world drug pyramid. You will be treated with more deference and sympathy than a junkie passing out on a subway car in Manhattan (using two seats of a subway car is a common prosecutable offense in this city). An international drug trafficker is a criminal and usually a murderer; the drug addict walking the street is one of his victims. But thanks to Breuer, we're now in the business, officially, of jailing the victims and enabling the criminals.
So there is absolutely no reason they couldn't all face criminal penalties. That they are not being prosecuted is cowardice and pure corruption, nothing else. And by approving this settlement, Breuer removed the government's moral authority to prosecute anyone for any other drug offense. Not that most people didn't already know that the drug war is a joke, but this makes it official.
The US is the world's largest prison state, imprisoning more of its citizens than any nation on earth, both in absolute numbers and proportionally. It imprisons people for longer periods of time, more mercilessly, and for more trivial transgressions than any nation in the west. This sprawling penal state has been constructed over decades, by both political parties, and it punishes the poor and racial minorities at overwhelmingly disproportionate rates.
But not everyone is subjected to that system of penal harshness. It all changes radically when the nation's most powerful actors are caught breaking the law. With few exceptions, they are gifted not merely with leniency, but full-scale immunity from criminal punishment. Thus have the most egregious crimes of the last decade been fully shielded from prosecution when committed by those with the greatest political and economic power: the construction of a worldwide torture regime, spying on Americans' communications without the warrants required by criminal law by government agencies and the telecom industry, an aggressive war launched on false pretenses, and massive, systemic financial fraud in the banking and credit industry that triggered the 2008 financial crisis.
Saturday, December 8, 2012
A Look at the Morphinan Structure Activity Relationships of Six Popular Opiates
The terms opiate and opioid are often used interchangeably, different authors define what is considered an opiate or an opioid with little consistency. The suffix "-iod" means like, hence opioids act like opiates. In general opiate refers to alkaloids found in the opium poppy that intereact with the body's endorphin receptors. Opioids are molecules that interact with the same receptors but are either fully synthetic (ie fentanyl), derived from the alkaloids found in opium and thus semi-synthetic (ie buprenorphine), and molecules from other plants that are distinct from morphine but nonetheless do interact with the opioid receptors (eg kratom). For the purposes of this blog, I define opiates are any molecules with only minor derivations on morphine. The following is a discussion of five common pharmaceutical narcotics and how they relate to the prototypical opiate, morphine. I consider codeine, hydromorphone, oxymorphone, hydrocodone and oxycodone all to be opiates. The most common changes to the morphine molecule involves:
1. Changing substituents at carbons 3 and 6. In morphine these are alcohol (-OH) groups.
2. Reduction of the double bond between carbons 7 and 8.
3. Addition of an alcohol (-OH). group at carbon 14.
4. Addition or changes to the group coming off the nitrogen, carbon #17.
Figure 1. Morphine with the carbon atoms numbered. Morphine is the primary alkaloid in opium.
 Codeine is also found naturally in opium, and in (slightly) more enlightened countries is sold over the counter, though never without added acetaminophen (Tylenol) or acetylsalicylic acid (aspirin). Codeine is identical to morphine but has a methyl group attached to the oxygen on carbon #3. A carbon-oxygen-carbon grouping is known as an ether, thus codeine is 3-methyl ether morphine. This dramatically reduces the activity of codeine to only 10% of morphine.
Codeine is also found naturally in opium, and in (slightly) more enlightened countries is sold over the counter, though never without added acetaminophen (Tylenol) or acetylsalicylic acid (aspirin). Codeine is identical to morphine but has a methyl group attached to the oxygen on carbon #3. A carbon-oxygen-carbon grouping is known as an ether, thus codeine is 3-methyl ether morphine. This dramatically reduces the activity of codeine to only 10% of morphine. Hydromorphone has two changes to the morphine molecule which increases its relative potency. The OH group at position 3 in morphine has the hydrogen removed, the oxygen is now double bonded to carbon 6. A carbon-oxygen double bond is known as a ketone ("key-tone"), thus the "-one" at the end of hydromorphone. The double bond between carbons 7 and 8 has been reduced to a single bond, by adding two hydrogen atoms (H not shown). This should make the molecule "dihydro-morphin-one," due to the addition of two hydrogen molecules (dihydro) and the oxidation of the OH group at carbon 6 to a ketone (morph-INE to morph-ONE). However the name is derived not from the double bond between carbon's 7 and 8, but for the atom bonded to carbon 14. In this case hydromorphone retains the same configuration as morphine, a single H at carbon 14.
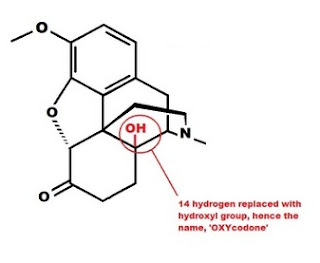
Hydromorphone has two changes to the morphine molecule which increases its relative potency. The OH group at position 3 in morphine has the hydrogen removed, the oxygen is now double bonded to carbon 6. A carbon-oxygen double bond is known as a ketone ("key-tone"), thus the "-one" at the end of hydromorphone. The double bond between carbons 7 and 8 has been reduced to a single bond, by adding two hydrogen atoms (H not shown). This should make the molecule "dihydro-morphin-one," due to the addition of two hydrogen molecules (dihydro) and the oxidation of the OH group at carbon 6 to a ketone (morph-INE to morph-ONE). However the name is derived not from the double bond between carbon's 7 and 8, but for the atom bonded to carbon 14. In this case hydromorphone retains the same configuration as morphine, a single H at carbon 14.  Oxymorphone has the same two changes to the morphine molecule as hydromorphone, but also has an OH group attached to carbon 14 in place of the hydrogen in morphine. This increases the potency and is the reason the name is OXY-morph-ONE. The oxy prefix refers to the OH on carbon 14, and the one suffix refers to the change at carbon 6.
Oxymorphone has the same two changes to the morphine molecule as hydromorphone, but also has an OH group attached to carbon 14 in place of the hydrogen in morphine. This increases the potency and is the reason the name is OXY-morph-ONE. The oxy prefix refers to the OH on carbon 14, and the one suffix refers to the change at carbon 6.
Both oxycodone and hydrocodone involve the same changes to the morphine molecule as oxymorphone and hydromorphone, but include the methyl (-CH3) group attached to the 3rd carbon just like codeine.

The changes to the morphine structure can be summarized as follows:
A. Addition of a methyl group to the oxygen on carbon 3. Creates a methyl-3-ether linkage. Reduces potency.
B. Alcohol group (-OH) on carbon 6 oxidized to a double bonded ketone (=O). Increases potency.
C. Hydrogenation (two H atoms are added) of double bond between carbons 7 and 8. Increases potency.
D. Substitution of an alcohol group (-OH) for the hydrogen at carbon 14. Increases potency.
Opiate Changes to Morphine Brand Names
Morphine - MS Contin
Codeine A Paramol, Tylenol 3
Hydromorphone B, C Dilaudid, Palladone
Oxymorphone B, C, D Opana, Numorphan, Numorphone
Hydrocodone A, B, C Vicodin, Lortab
Oxycodone A, B, C, D Oxycontin, Percocet, (More here)
 Oxycodone is an example of all four changes to the basic morphine structure. A decreases the potency, while B, C and D increase the potency. The net result is a molecule slightly more potent than morphine, though far less potent than oxymorphone.
Oxycodone is an example of all four changes to the basic morphine structure. A decreases the potency, while B, C and D increase the potency. The net result is a molecule slightly more potent than morphine, though far less potent than oxymorphone.  Narcotic antagonists are made by using the oxymorphone structure with modified substituents on the nitrogen. The groups off the nitrogen have major effects on the pharmacological activity. Both naloxone and naltrexone are used to reverse opioid overdoses and have no intrinsic opioid activity of their own.
Narcotic antagonists are made by using the oxymorphone structure with modified substituents on the nitrogen. The groups off the nitrogen have major effects on the pharmacological activity. Both naloxone and naltrexone are used to reverse opioid overdoses and have no intrinsic opioid activity of their own. Friday, December 7, 2012
Cannabis Legalization, Good or Bad?
If weed has 100% legalized everywhere in the world the drug wars could continue. How many people are busted for weed each year? Something like 800,000? That number could easily be replaced with the ranks of other drug users.
The worst case scenario, as I see it, following cannabis legalization would be a shift in focus to other drugs with no change in enforcement dollars or priorities. In the absence of marijuana, there’s always coca and poppy crops to be eradicated, dealers and users to be hunted down and locked up.
I think this is unlikely, though possible. In part because the debate over weed has included the notion that there are costs to prohibition, costs which are due to prohibition and not drugs (a distinction that prohibitionists do their best to obfuscate). Prohibition has failed at eliminating people’s access to these drugs, a failure which is all the more remarkable given its long history of failure and official denial (that a drug free America is possible, or even desirable). Places like South America, which have born the brunt of the drug war, have had enough.
I think this is unlikely, though possible. In part because the debate over weed has included the notion that there are costs to prohibition, costs which are due to prohibition and not drugs (a distinction that prohibitionists do their best to obfuscate). Prohibition has failed at eliminating people’s access to these drugs, a failure which is all the more remarkable given its long history of failure and official denial (that a drug free America is possible, or even desirable). Places like South America, which have born the brunt of the drug war, have had enough.
Legalizing heroin may be too radical a notion for the masses who have known nothing but propaganda for over a generation (and I understand this since I was a DARE grad and firmly anti-drug until being exposed to alternative views), but I think a possible chip in the established dogma would be to start with the plants. Poppies and coca have been used for a very long time, the right to cultivate and use these plants is an easier sell than heroin and cocaine.
Sunday, December 2, 2012
Chirality, a Primer (Updated)
 The word chirality comes from the Greek word for "handedness," as in left or right-handed. Well it turns out molecules can also have different forms that are analogous to the idea of handedness. For an example look at alanine. The figure shows two versions, or what chemists call enantiomers, of the amino acid alanine. Much like each hand has five fingers, one thumb, ect both molecules have the same number of atoms and same general chemical structure, but make no mistake these are two distinct compounds.
The word chirality comes from the Greek word for "handedness," as in left or right-handed. Well it turns out molecules can also have different forms that are analogous to the idea of handedness. For an example look at alanine. The figure shows two versions, or what chemists call enantiomers, of the amino acid alanine. Much like each hand has five fingers, one thumb, ect both molecules have the same number of atoms and same general chemical structure, but make no mistake these are two distinct compounds.
Chemists have different ways of naming the different enantiomers. There are three different systems for naming, the S/R system is preferred by chemists and involves numbering the atoms bonded to the center of the plane of symmetry (the chiral carbon). The +/- system is based on how each enantiomer shifts light, either in a (+) dextrorotary (clockwise), or (-) levorotary (counterclockwise) direction. Finally there is the D/L system of naming, which has nothing to do with light and is based on the labeling of the biological molecule glyceraldehyde. Confused yet? Me too. All you really need to know is that these are two distinct molecules.
Chirality is an important chemical concept, particularly for pharmacology and medicinal chemistry. Most drugs work by interacting with a specific receptor, oftentimes described like a key (drug molecule) fitting into a lock (receptor). It is not uncommon for receptors to respond to only one of the two forms, if the receptor was a left-handed glove it would fit well with a left hand but not very well with a right hand. One of the most famous examples of different pharmacological effects due to chirality is methamphetamine.
Methamphetamine has two enantiomers, L-meth and D-meth. D-methamphetamine is the good stuff, well I personally wouldn't touch the stuff as coffee gets me as stimulated as I care to be, but D-meth is what speed aficionados like. L-meth is much weaker with little effect on the central nervous system, although it is a good decongestant and is even used in Vicks inhalers!
When drugs are synthesized in a laboratory, the chemical reactions often result in a 50:50 mix of the enantiomers, called a racemic mixture. Because the two drugs have the same basic chemical structure, they are share physical properties (solubility, melting point, ect), which makes them difficult to separate. In some cases, such as virtually all the methadone sold in the US, what people are actually getting is two different drugs with different pharmacological properties.
 Methadone such as is dispensed at clinics across the US, is a 50:50 mix of (S)-Methadone and (R)-Methadone. The only difference is the direction the methyl (CH3) group is facing, in the diagram to the left, in (S)-methadone the methyl is pointing forward as signified by the solid black line. In (R)-methadone the methyl is facing away from the viewer, into the page as signified by the dashed line. For another view of the two enantiomers see the three-dimensional methadone models in the image. The white arrow points to the methyl group, otherwise the molecules are identical.
Methadone such as is dispensed at clinics across the US, is a 50:50 mix of (S)-Methadone and (R)-Methadone. The only difference is the direction the methyl (CH3) group is facing, in the diagram to the left, in (S)-methadone the methyl is pointing forward as signified by the solid black line. In (R)-methadone the methyl is facing away from the viewer, into the page as signified by the dashed line. For another view of the two enantiomers see the three-dimensional methadone models in the image. The white arrow points to the methyl group, otherwise the molecules are identical.  The position of that methyl has a large effect on methadone's opioid properties. For all practical purposes, only (R)-methadone actually has any opioid effect. One possible explanation for this has to do with the space between the nitrogen (blue in model) and the oxygen (red). In (R)-methadone the methyl is facing away from the space between the oxygen and nitrogen, which allows a bond to form. In (S)-methadone the methyl group is blocking this space.
The position of that methyl has a large effect on methadone's opioid properties. For all practical purposes, only (R)-methadone actually has any opioid effect. One possible explanation for this has to do with the space between the nitrogen (blue in model) and the oxygen (red). In (R)-methadone the methyl is facing away from the space between the oxygen and nitrogen, which allows a bond to form. In (S)-methadone the methyl group is blocking this space.  It should also be noted that not all molecules have mirror images, hence they are achiral. Other molecules have more than one chiral center, and the number of different combinations of enantiomers (called stereoisomers) grows exponentially. For example morphine has five chiral carbons, marked with red dots. Theoretically at least, morphine has 32 different stereoisomers. Fortunately plants (and animals) are masters of biochemistry and are able to produce a single specific form of morphine, as all the different stereoisomers could have different pharmacological effects. This is also a reason why poppies are still used as the source material for all opiates currently used in medicine today.
It should also be noted that not all molecules have mirror images, hence they are achiral. Other molecules have more than one chiral center, and the number of different combinations of enantiomers (called stereoisomers) grows exponentially. For example morphine has five chiral carbons, marked with red dots. Theoretically at least, morphine has 32 different stereoisomers. Fortunately plants (and animals) are masters of biochemistry and are able to produce a single specific form of morphine, as all the different stereoisomers could have different pharmacological effects. This is also a reason why poppies are still used as the source material for all opiates currently used in medicine today. Update, 12/3/2012
If you are taking methadone what does this mean for you.? If you live in the US, and probably most of the world (there may be places where you can get enantiomerically pure (R)-methadone, I just am not aware of any) you are getting a racemic (50:50) mixture of R/S Methadone. (R)-methadone is 10-50 times stronger than (S)-methadone, so for all practical purposes only half of your dose is actually an opioid.
This becomes relevant clinically because the different enantiomers have different pharmacological properties. Take peak-and-trough testing for example. Let's say you are on a high dose of methadone but wake up every morning feeling sick. You request an increase but since you are already on a high dose the clinic says, "Hold up there we can't take your word for it, you're a junkie and everyone knows junkies lie so we are going to confirm your feelings with a blood test."
Peak and trough testing is a blood test that measures the level of methadone at two points in a 24 hour period. The first is right before dosing when the methadone is lowest (the trough), then another blood test occurs about 3 hours after dosing when the methadone has reached maximum blood concentration (the peak). Supposedly based on this test they can tell if you really need additional methadone.
But here's the thing, the blood test does not distinguish between (S)- or (R)-methadone. While they are similar in structure and share many pharmacological properties, they are not the same. An individual may metabolize (R)-methadone faster than (S)-methadone, so that by the time they wake up in the morning the level of (R)-methadone is way down and they are beginning to feel withdrawal. However their blood is still loaded with (S)-methadone, so the peak and trough test, which does not distinguish between R and S enantiomers, gives results that indicate the level of methadone is adequate. And so the doctor with their test results can tell the patient it's all in their head, when in reality there is a clinical reason why the person wakes up every morning feeling ill. The patient should always be trusted when it comes to their own body.
There may be other differences in the two enantiomers. Methadone has action on other receptors besides the endorphin receptors. Would there be any advantages to using pure (R)-methadone? At this point I am not sure but will revisit this issue as I learn more.
Read more about Peak and Trough testing here.
Subscribe to:
Posts (Atom)







